[Courtesy of UNIST]
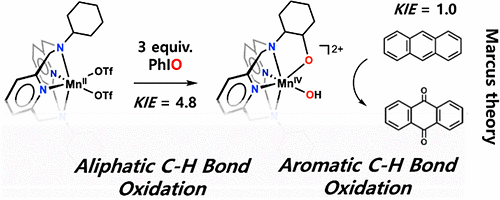
Anthracene is a polycyclic aromatic hydrocarbon and some investigations indicate that it has carcinogenic and gene toxicity characteristics. The hydrocarbon that causes air pollution is composed of three benzene rings. Because benzene has a very strong structure and it is very hard to break the bonds between atoms, anthracene is chemically stable with low solubility.
Because of the stable structure of anthracene, the effective dissociation of the hydrocarbon is regarded as an important milestone in the environmental biochemical industry. To dissociate anthracene, a high temperature and high pressure environment is required.
The Ulsan National Institute of Science and Technology (UNIST) said that its researchers worked with Jana Roithova from Netherland's Radboud University to find that a high-valent manganese hydroxo complex effectively dissociated the toxic hydrocarbon material through a process based on the Marcus theory of electron transfer.
Researchers synthesized the metal dioxidase after a similar metallic enzyme that can dissociate naphthalene, an organic hydrocarbon compound commonly known as mothballs. "We have found a way to dissociate polycyclic aromatic hydrocarbons by designing a catalyst that does not need high pressure or high temperature," UNIST researcher Lee Yu-jeong said in a statement on November 28.




![[CES2024] Doosan Group showcases cooperative robots and zero-carbon tech at CES](https://image.ajunews.com/content/image/2024/01/09/20240109141547385883.jpg)