[Courtesy of UNIST]
SEOUL -- A team of researchers from a state research institute has developed a cheap metal-based catalyst that has about 200 percent higher efficiency in water electrolysis, the electrochemical breakdown of water into hydrogen and oxygen, than precious metal-based electrodes.
Electrolysis that uses electricity to break down water is the core technology for the production of green hydrogen, which is hydrogen fuel produced only using renewable energy. However, such a method requires expensive catalysts made using precious metals such as palladium or platinum. The high price of the components makes green hydrogen expensive, making it less attractive to consumers who are considering whether to choose fossil fuel or hydrogen fuel to operate their vehicles or factories.
To increase the efficiency of the electrolysis process, an anion exchange membrane water electrolysis cell system was developed. The system requires ultrapure water to achieve maximum efficiency and requires more energy than other electrolysis systems that break down alkaline or acidic water. Also, the newly-developed electrolysis system requires special catalysts. Ultrapure water with zero impurities including minerals and other additional chemical substances is required by the anion exchange membrane water electrolysis cell system to maximize the lifespan of facilities.
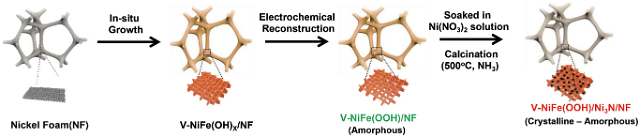
The Ulsan National Institute of Science and Technology (UNIST) said that its research team manufactured a non-precious metal-based high-performance oxygen generation catalyst by growing nickel nitride on the surface of vanadium-nickel-iron oxide-hydroxide through electrodeposition and nitridation processes. Because iron oxide-hydroxides have low conductivity, researchers doped it with vanadium and electrochemically reconstructed nickel on the surface of the electrodes.
Because nickel is fifth most abundant element on Earth, it is very affordable. "It is vital to achieve the performance and stability of the anion exchange membrane water electrolysis cell technique in order to commercialize it," UNIST researcher Kwon Young-kook said in a statement on February 21. The newly-developed catalysts were able to stability maintain their operability for one thousand hours without damage. The electrodes showed about 200 percent higher electrolysis performance than conventional electrodes made of precious metals.




